Le Chatlier's Pranciple | Effect of concentration | Effect of Pressure| Effect of Temperature
Description:
Welcome to "Le Chatelier's Principle: Exploring the Effects of Concentration, Pressure, and Temperature." In this video, we delve into the fundamental concepts of chemical equilibrium and investigate how changes in concentration, pressure, and temperature influence the equilibrium position of a reaction, as described by Le Chatelier's Principle.
Firstly, we'll explore the effect of concentration on a system at equilibrium. By adding or removing reactants or products, we can disrupt the equilibrium and observe the system's response as it adjusts to restore equilibrium. Through visual demonstrations and explanations, we'll illustrate how changes in concentration shift the equilibrium position, ultimately reaching a new balance.
Next, we'll examine the impact of pressure changes on equilibrium. For gaseous reactions, alterations in pressure can significantly affect the equilibrium state. By altering the volume of the container or adjusting the number of moles of gas involved, we can observe how the equilibrium shifts to counteract changes in pressure, following Le Chatelier's Principle.
Finally, we'll investigate the influence of temperature on chemical equilibrium. Temperature changes can either favor the forward or reverse reaction, depending on whether the reaction is exothermic or endothermic. We'll demonstrate how altering the temperature affects the equilibrium constant and explore the factors influencing this dynamic equilibrium.
Throughout the video, we'll provide detailed explanations, real-life examples, and visual representations to help you grasp the intricate interplay between concentration, pressure, and temperature on chemical equilibrium, as governed by Le Chatelier's Principle.
Join us on this educational journey as we unravel the mysteries of equilibrium and deepen our understanding of Le Chatelier's Principle. Don't forget to like, share, and subscribe for more insightful content on chemistry and science!
-
 3:35
3:35
Veritasium
19 days agoSupercooled Water - Explained!
4 -
 8:37
8:37
Veritasium
19 days agoThe Inverse Leidenfrost Effect
7 -
 8:11
8:11
Veritasium
19 days agoCelsius Made His Thermometer Upside Down
4 -
 1:05
1:05
Veritasium
19 days agoExperiments A Cappell
4 -
 0:37
0:37
Veritasium
19 days agoChain Drop Experiment
4 -
 1:31
1:31
Veritasium
19 days agoWhat Is Chemistry?
6 -
 19:31
19:31
Math Easy Solutions
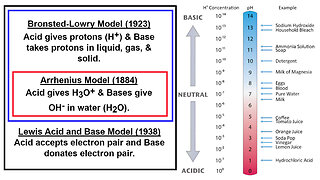
24 days ago $0.07 earnedAcids and Bases: Brønsted–Lowry, Arrhenius, and Lewis Models
1571 -
 55:03
55:03
PsychReviews2
1 month agoAnalytical Psychology: The French School
40 -
 2:13
2:13
The Ball Is Wild
2 months agoBOM Manipulated Temperature Records To Show False Temperature Increase
112 -
 0:10
0:10
RM Awesome Randoms
1 month agoThe "anchoring effect" affects...
3