The Walden Inversion and the SN2 Mechanism. Help me with Organic Chemistry!
This video will explain why the SN2 mechanism results in the complete inversion of stereochemistry. (S to R or R to S). The Walden inversion occurs when a nucleophile attacks from the back side of an electrophilic carbon. As the nucleophile attacks the leaving group begins to leave. The nucleophile bond forms at the same rate the leaving group leaves. As a result of this the electrophilic carbon goes from a tetrahedral carbon to a trigonal bipyramid transition state back to a tetrahedral carbon.
Failing organic chemistry? You do not have to fail Organic Chemistry!
This video is part of a series called How to be Successful in Organic Chemistry. In this series I go over numerous problems that a student could expect to see in there organic chemistry 1 course. Doing organic chemistry practice problems will make you more successful in organic chemistry and biochemistry.
I recommend that you download the problem from the link below and attempt the problem yourself and use this video to correct your work.
Download the problem from this video at the following link:
https://www.dropbox.com/s/mmk12jyehkvcqn1/Why%20Does%20the%20Stereochemistry%20of%20an%20SN2%20Reaction%20Invert%3F%20%20Walden%20Inversion%3F.pdf?dl=0
Good Luck and Good Chemistry!
Please subscribe to my channel by clicking the link below!
https://www.youtube.com/c/AllInwithDrBetts?sub_conformation=1
Like this video and leave a comment below!
-
 2:38
2:38
Chemistry Tutor
1 year agoPre Nursing Student Dosage Calculation for Metoprolol: A Guide for Pre Nursing Students
31 -
 2:38
2:38
Chemistry Tutor
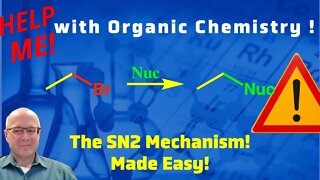
1 year agoThe Mechanism of the SN2 Reaction Video Help Me With Organic Chemistry!
45 -
 3:26
3:26
Chemistry Tutor
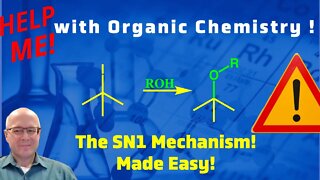
1 year agoThe Mechanism of the SN1 Reaction Video! Help Me With Organic Chemistry!
47 -
 7:43
7:43
Chemistry Tutor
1 year agoThe Mechanism of an SN1 Reaction With a Rearrangement Video Help Me With Organic Chemistry!
20 -
 3:45
3:45
Chemistry Tutor
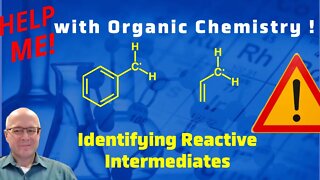
1 year agoIdentifying Reactive Intermediates - Help Me With Organic Chemistry!
15 -
 4:56
4:56
Chemistry Tutor
1 year agoThe Mechanism of an SN1 Reaction With a Ring Expansion Video Help Me With Organic Chemistry!
15 -
 5:52
5:52
Chemistry Tutor
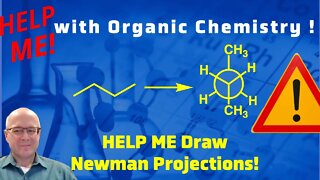
1 year agoNewman Projection of 2,3-dimethylbutane Help Me With Organic Chemistry!
20 -
 8:21
8:21
Chemistry Tutor
1 year agoHow to Draw a Newman Projection? Help Me With Organic Chemistry!
3 -
 5:31
5:31
Chemistry Tutor
1 year agoWhy does an SN1 Reaction Form Racemic Products? The SN1 Mechanism. Help Me With Organic Chemistry!
25 -
 6:52
6:52
Chemistry Tutor
1 year agoHyperconjugation and the Stability of Carbocations - Help Me With Organic Chemistry!
2