Why does an SN1 Reaction Form Racemic Products? The SN1 Mechanism. Help Me With Organic Chemistry!
If a chiral alkyl halide under goes an SN1 reaction it will deliver a racemic mixture of products. This video will explain how this occurs by drawing the mechanism of the SN1 reaction (SN1 mechanism) and examining the carbocation reactive intermediate. The carbocation intermediate has a trigional planer molecular shape. This means that nucleophilic approach can occur from the top or from the bottom. This results in both the R and S enantiomers forming and the product being racemic.
Failing organic chemistry? You do not have to fail Organic Chemistry!
This video is part of a series called How to be Successful in Organic Chemistry. In this series I go over numerous problems that a student could expect to see in there organic chemistry 1 course. Doing organic chemistry practice problems will make you more successful in organic chemistry and biochemistry.
I recommend that you download the problem from the link below and attempt the problem yourself and use this video to correct your work.
Download the problem from this video at the following link:
Good Luck and Good Chemistry!
Please subscribe to my channel by clicking the link below!
https://www.youtube.com/c/AllInwithDrBetts?sub_conformation=1
Like this video and leave a comment below!
-
 5:58
5:58
Chemistry Tutor
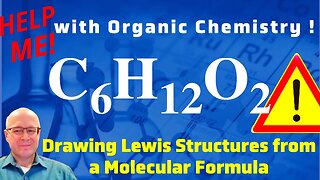
1 year ago $0.02 earnedHow to Draw a Lewis Structure From a Molecular Formula C6H14O2 Help with Chemistry!
54 -
 3:26
3:26
Chemistry Tutor
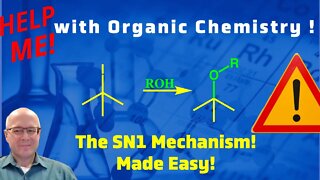
1 year agoThe Mechanism of the SN1 Reaction Video! Help Me With Organic Chemistry!
47 -
 2:38
2:38
Chemistry Tutor
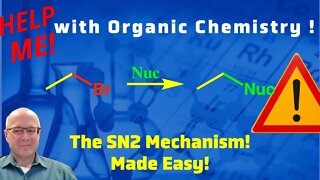
1 year agoThe Mechanism of the SN2 Reaction Video Help Me With Organic Chemistry!
45 -
 7:43
7:43
Chemistry Tutor
1 year agoThe Mechanism of an SN1 Reaction With a Rearrangement Video Help Me With Organic Chemistry!
20 -
 4:56
4:56
Chemistry Tutor
1 year agoThe Mechanism of an SN1 Reaction With a Ring Expansion Video Help Me With Organic Chemistry!
15 -
 5:00
5:00
Chemistry Tutor
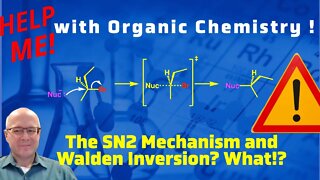
1 year agoThe Walden Inversion and the SN2 Mechanism. Help me with Organic Chemistry!
21 -
 3:45
3:45
Chemistry Tutor
1 year agoIdentifying Reactive Intermediates - Help Me With Organic Chemistry!
15 -
 5:52
5:52
Chemistry Tutor
1 year agoNewman Projection of 2,3-dimethylbutane Help Me With Organic Chemistry!
20 -
 8:21
8:21
Chemistry Tutor
1 year agoHow to Draw a Newman Projection? Help Me With Organic Chemistry!
3 -
 6:52
6:52
Chemistry Tutor
1 year agoHyperconjugation and the Stability of Carbocations - Help Me With Organic Chemistry!
2